IPM: An Emerging Strategy for Diaprepes in Florida Citrus
Clayton W. McCoy and Larry W. Duncan
University of Florida, IFAS
Citrus Research and Education Center
700 Experiment Road, Lake Alfred, Florida 33850
Phone: (863) 956-1151 Ext. 241
Fax: (863) 956-4631
E-mail: cwmy@icon.lal.ufl.edu
The Diaprepes root weevil, Diaprepes abbreviatus L., is one of many tropical species of root weevils known to reproduce on and damage citrus in the Caribbean region (Table 1). Larval injury to the roots by weevils cause an estimated annual loss of $ 75-100 million in citrus production as a result of tree mortality and yield reduction in commercial citrus in the Carribean region including Florida. This invasive species to Florida has spread to 22 counties producing citrus commercially during the past 36 years. The adult, egg, and neonate stages appear on the host plant above ground and all larval stages, and the pupa and teneral adult occur below ground. Initially, neonates feed on the small fibrous roots, but as they increase in size, they feed on the bark of increasingly larger roots. Later instars can remove the outer bark from the crown area of the root system or girdle the trunk found below the soil surface, thereby killing the tree. Larval injuries by D. abbreviatus serve as preferred infection courts for root rot diseases of citrus caused by soil pathogens such as Phytophthora spp. The interaction between root weevils and soil-borne fungal pathogens of the roots results in one of the most severe decline syndromes affecting citrus. Therefore, pest management of Diaprepes and other root weevils can require treatment of both the insect and soil-borne diseases.
There is a need to implement a decision support system for Diaprepes and other root weevils with emphasis on detection, threshold-based remedial actions and a mix of suppression strategies including the judicious use of pesticides when necessary. Our overall goal is to stabilize pest populations below economically damaging levels thereby preventing further spread while minimizing the impact on the environment.
The Problem
A repertoire of historical and contemporary methods for managing Diaprepes and other root weevils have been attempted or are in use today. They include numerous cultural, mechanical, natural, sanitary, biological and chemical methods for the suppression of different developmental stages. Although many of these methods contribute to weevil suppression, neither researchers nor most growers consider current suppressive tools to be adequate for economic management. There is no published research on the profitability of one or a combination of these programs that may or may not include disease control in the soil. The research constraints to answering these questions of field efficacy and profitability are tremendous. No methods exist to directly assess larval populations in soil and adult monitoring methods, though functional, are inefficient and are not grower friendly. Root loss and root damage can not be assessed without destruction of the tree. Root injury/yield relationships are complex, particularly when soil-borne fungal diseases are a factor. The role of alternate host plants in the weevil biology and field ecology of the Diaprepes root weevil is poorly understood, making it difficult to initiate management strategies for all development stages of the weevil.
Cultural Control
Improving soil drainage appears to be the most fundamental strategy for minimizing root injury caused by the larvae. Drainage ditches and raised planting beds in lowland reduce the amount of standing water in orchards and lower the water table. As a result, the rhizosphere is larger and better-drained and favors rapid, healthy root growth free of water stress and disease. Although citrus root weevil larvae feed readily on roots growing under these improved conditions, the tree generates new roots at a more rapid rate so that less overall tree decline occurs over time. It has been observed but not proven scientifically that improved drainage also appears to reduce the incidence of diseases caused by soil-borne fungi associated with larval injury to roots. The combination of root disease and larval feeding can be devastating to a tree. Where drainage has been improved in declining groves, the regular use of a combination soil fungicide-insecticide can significantly improve canopy leaf density and productivity, particularly when declining trees are also rejuvenated by aggressive canopy pruning and regular tree care.
Bare rooting is another method of limiting crown injury caused by Diaprepes larvae, practiced in the Carribean and occasionally in Florida. The upper 4-6 inches of soil is removed by means of water under pressure, in an area extending 2 feet from the base of the trunk, to expose the crown region of the root system thereby prevent larval feeding on this vulnerable part of the tree. This tactic has been used mainly to prevent tree mortality in declining young groves. Unless combined with effective control of adults and larvae on other parts of the tree, this labor-intensive practice will likely fail.
Fertigation at monthly intervals has been successfully used by a few growers in Florida to promote the growth of fibrous roots near the soil surface after weevil larvae have destroyed the taproot and inner crown of the tree. The promotion of fibrous root growth can significantly improve tree health and average fruit production in declining groves. This strategy also will be effective for only a short time unless weevil adults and larvae are controlled by other means.
Although skirt pruning has been effectively to control the flightless Fuller rose beetle, it would appear to have little effect on Diaprepes, who can readily fly from tree to tree.
Cover cropping has been practiced in a number of countries in the Carribean, however, it is difficult to determine its success from the literature. Early in the century, the leguminous plant Tephrosia candida was planted between rows or around citrus trees to repel adult Diaprepesspecies, but it is no longer used as a cover crop. Observations in Jamaica suggest that citrus root weevil adults are attractedto Gliricidia species (quick stick) more readily than to adjacent citrus trees, so that quick stick may have potential as a trap crop. However, other reports suggest that it attracts Diaprepes species to citrus. Further research should resolve this contradiction reported in the literature. In Puerto Rico, another leguminous plant, pigeon pea, appears to be effective as a trap crop for adultsDiaprepesspecies around citrus plantings.
In the past, deep tillage was practiced in the Caribbean to expose larvae to predators in the soil beneath the tree. However, mechanical root injury to the tree is always a concern when using this practice. This cultural method is rarely used today and is impractical in most commercial citrus-growing areas.
Mechanical Control
In the Caribbean regions, where agricultural labor is less expensive than in the United States, the large, adult Diaprepes are conspicuous enough in the tree canopy for hand-picking. Teams of workers can hand-collect weevils from leaves or from white cloth sheets spread beneath the canopy, which catch weevils that fall from the tree when the branches are shaken. The weevils are usually collected in containers and then killed in a kerosene bath or burned. The economic benefit gleaned using this mechanical control to reduce adult populations is unknown.
In parts of the Caribbean and in Florida, some growers spread strips of black plastic, approximately 3 feet wide, on the soil on opposite sides of the trunk beneath the canopy to trap newly hatched larvae as they drop from the leaves. During the heat of the day, larvae can be killed within minutes as they land on the hot plastic, or they are forced away from the tree to the row middle, where the are killed on the hot, dry soil or consumed by predators. When used over a large area, this mechanical barrier can aid weed control, but it causes problems in routine grove maintenance, particularly irrigation and fertilization. Wind can also be a limiting factor in maintaining an effective barrier.
In summary, cultural and mechanical control methods have been grower-driven and lack empirical data to support observations.
Natural Control
A wide range of parasites, predators and pathogens attack the Diaprepes root weevil at one or more developmental stages within the tree canopy or in the soil. Most or these natural enemies appear to be widely distributed and are general feeders such as birds, spiders, ants, nematodes and microbes in the soil. As Dr. Pena pointed out earlier, the hymenopterous egg parasitoids differ widely and are quite host-specific. Quadrastichus haitiensis is most widely distributed on citrus and is being introduced into Florida as a classical biological control agency ofDiaprepes in Florida. Virtually nothing is known about the population dynamics of these egg parasitoids and their potential as regulatory agents in the field; however, reports of high egg parasitism in some locations have been published.
Both invertebrate and vertebrate predators are known to feed on adult weevils during the arboreal portion of their life cycle. Birds, reptiles, spiders, ants, and mammals have been observed preying on adult weevils. As a previous speaker pointed out, tree-inhabiting ants, particularly predatory species in the genera Monomorium and Crematogaster, and various spiders have been observed consuming eggs of citrus root weevils. The importance of egg and adult predation is unknown, and no attempts have been made to rear and release these predators as biological control agents or to augment their population in the field by environmental manipulation.
Numerous general predators, such as ants, ant lions, earwigs, and mites, forage for neonates on the soil surface beneath the tree canopy. Some larvae can repel ant predators by releasing defense pheromones. On small farms in many Carribean countries, chickens and guinea hens are kept in groves to reduce larval numbers. Predation by insects, mites, and vertebrates can have a significant effect on larval populations, and it may be possible to enhance this form of natural control of weevil larvae by environmental manipulation.
Citrus soils around the world contain microscopic nematodes that are obligate parasites of soil insects. Native species, as infective juveniles, penetrate through external openings of the body of emerging adults citrus root weevils and, more often, larvae in all stages in the soil. Although ecological data on this subject is limited, it would appear that a complex of natural-occurring nematodes species can exhibit a high level of natural control of larvae during the summer months.
Fungal and protozoan pathogens have been found attacking both adult and larval stages of citrus root weevils. Entomopathogenic fungi are ubiquitous in nature, while unicellular gregarines and microsporidial protozoans appear more specific. Viral and nonmutualistic bacterial infections have not been described. In Florida citrus soils, entomopathogenic fungi appear to be most prevalent before the summer rainy season.
While entomopathogenic fungi and entomogenous nematodes can reduce weevil population in the soil under natural conditions, their spatial distribution and persistence in the soil is variable. Soil texture, soil temperature, soil moisture, ultraviolet light, and various natural and artificial antagonists are important abiotic and biotic factors that influence populations in space and time. Research on biological control is therefore focusing on the supplementation of naturally-occurring nematodes that are amenable to mass production and are applied as biopesticides for short-term pest control.
Chemical Control
Chemical control has been used to reduce populations of citrus root weevil larvae and adults in the field. Over 30 different chemicals have been applied as 1) foliar sprays to control adult weevils, 2) soil drenches to control invasive neonates, and 3) granules or fumigants in soil treatments to control larvae and emergent adults.
Until their cancellation, the chlorinated hydrocarbons, aldrin and dieldrin, were widely used as a soil treatment to kill neonates. In recent years, organochlorines, carbamates and on occasion even certain nematicides (Nemacur, Mocap) have been used against larvae and adults, but residual control has generally been short and unreliable.
Petroleum oil is widely used for both arthropod and disease control in Florida citrus. When sprayed on citrus foliage alone or in combination with another pesticide, medium petroleum oil appears to affect the bonding characteristic of the substance holding the leaves together around an egg mass. It alters the protection provided by the folded leaf and exposes the egg mass, thus increasing the natural mortality of the eggs through predation and desiccation.
Foliar Treatment
Four chemicals are recommended as foliar sprays to reduce adult populations in the tree during peak emergence from the soil. Various formulations of Sevin (80S, 4F + XLR) are highly effective as a contact adulticide at 8 lbs ai/A, however, residual control is generally less than 75% after 3 wk post treatment. Guthion 50WP at 4 lbs/A and Orthene 755 at 5.22 lbs/A are similar to Sevin as to mode of action and residual control. However. Orthene can be used on non- bearing trees only. The purpose of adult suppression is to limit the number of gravid females and egg deposition, thereby reducing the number of larvae entering the soil. Foliar sprays should include a spreader-sticker adjuvant, such as medium oil, to improve coverage of new flushes. Because of their short residual activity, two sprays are necessary during the peak emergence period, which can last 10-12 weeks. Multiple pesticide applications, particularly during the summer, can interfere with the efficacy of natural enemies. Therefore, foliar sprays should be applied only when adults of the most important species are readily visible on the foliage or at peak catch in ground traps.
The insecticide growth regulator, Micromite (diflubenzuron) is recommended for use in the spring and summer for citrus rust mite and citrus leafminer control respectively in Florida citrus. When female Diaprepes feed on treated leaf flush, within a few days, only non-viable eggs are produced by females for as long as the residue is biologically active on the leaf (about 3 wk). In addition, existing egg masses laid prior to or after application that are exposed to the chemical directly or via the leaf residue will have reduced egg viability. Growers should be aware of this benefit from Micromite, when using the product as an acaricide or insecticide.
Soil Barrier Treatment
Capture 2EC (bifenthrin) is recommended as a soil treatment at 0.50 lbs ai/A for controlling invasive Diaprepesneonates in the spring under a Section 18 Emergency Exemption for citrus. Application should be made about 2-3 wk after the beginning of spring adult emergence from the soil. Application should be by ground equipment (herbicide-type spray boom) to bare soil beneath the tree at a minimum of 30 gal. per acre. Preferably, soil should be moist prior to application. Research suggests that about 80% or greater of the invasive neonates are killed or prevented from entering the root zone. Although Capture 2EC will suppress fire ant population (Solenopsis invicta), other predatory ants appear less effected and invade niches previously dominated by S. invicta.
Biological Control
As Dr. Duncan pointed out in the previous paper, three species of entomopathogenic nematodes are now available commercially by private industry. Preparation of S. riobrave, H. indica and H. bacteriophora are currently the only soil treatments used for the control of all developmental stages of Diaprepes larvae in Florida. They are applied at a recommended rate of 200 million/A, regardless of tree age and planting density. Obviously, a rate per area recommendation results in some variation in number applied beneath the tree based on area treated. Nematodes should not be applied within 4 weeks of nematicide use.
Sampling Methods for Diaprepes Larvae and Adults
Quantitative sampling methods are vital to the development of a decision support system forDiaprepes and other root weevils. Sampling programs should emphasize threshold based remedial actions using various non chemical and chemical IPM tactics. Since Diaprepes has numerous weed hosts associated with citrus, where and how to sample for larvae and adults is a major issue. Larvae are protected by the roots so that soil probe or auger methods are virtually impossible to implement. Research using acoustic detection of noises generated during larval feeding is still in its infancy. Polyethylene plastic funnel traps have been used as a research tool to catch neonates dropping to the soil upon hatching from the egg; however, trap catches have not been correlated with subsequent larval survival in soil.
The simplest way for a grower to determine the presence of adult Diaprepes in a citrus grove is to examine the most recent leaf flush for the typical notch-like feeding pattern along the margin of the leaves. If notching is present, then examine the same foliage for adult weevils and/or egg masses. Since weevils are nocturnal, the best time to scout for adults is in the early morning or late evening hours on outer portions of the tree. When the adults are disturbed they will usually fall to the ground, feigning death. For easier detection a "beating apron" may be used to catch the adults as they fall to the ground.
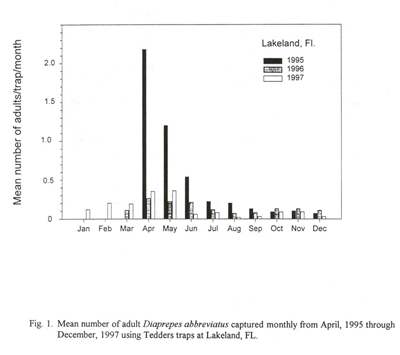
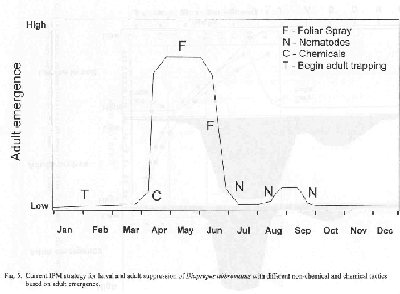
By regularly monitoring adult emergence from the soil, the estimated time and intensity of seasonal occurrence in the grove can be determined for citrus, although adult emergence from adjoining host plants could alter the typical emergence cycle slightly. By knowing the species of weevil and their emergence pattern from soil, a grower can make assumptions on when to apply larval and adult control measures (Fig. 1).
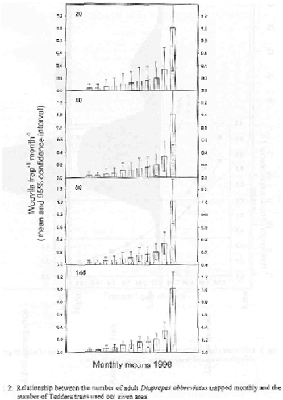
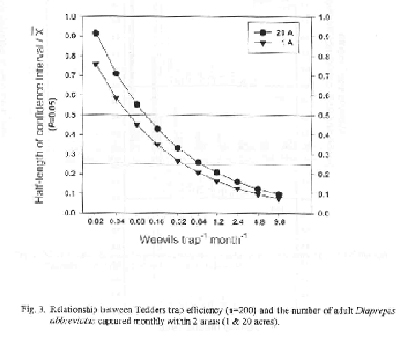
Various light traps, electrocutor funnel traps, sticky traps and ground traps of many shapes and sizes have been designed and tested in the field to monitor adult emergence. Although many are capable of capturing adults upon emergence from the soil, a 3 ft dark colored unbaited pyramidal trap capped with detachable concial top (Tedders trap) is most effective in trapping adults found on the soil beneath the canopy of the tree. (See Appendix for details on how to purchase the Tedders traps). When placed singly beneath the tree, midway between the dripline and trunk, about 80 Tedders traps distributed randomly within a given area (1-20 acre) will capture enough adults monthly to detect significant changes in seasonal fluctation in adult population on the soil beneath the tree, (Figs. 2 & 3). Research suggests that 1) seasonal adult capture for Tedders traps compared to cone shaped ground traps are similar, 2) Tedders trap efficiency is not affected greatly by geographical location (flatwood and ridge) and size of grove area, and 3) assuming the use of 200 traps per plot and Diaprepes abundance of 0.08 weevils/trap/month, the sampling precision is approximately 0.50 and varied 10 percentage points between plots of different size (Fig. 3). For 200 traps, and an abundance of 0.64 weevils/trap/month, the sampling precision varied 5 percentage points among the various plot sizes and is < 0.25. Sampling sizes ≥ 100 Tedders traps are estimated to provide sampling precision < 0.50 at D. abbreviatus abundance > 0.32 weevils/trap/month and < 0.25 at weevil abundance > 1.2/trap/month (data not shown). Since Tedders traps appear efficient enough to measure seasonal adult emergence, it would appear that the deployment of monitoring locations in different regions of the state as a pest alert program, should be a valuable input to assisting the grower in making IPM strategies each year.
Current IPM Strategy for Diaprepes in Citrus
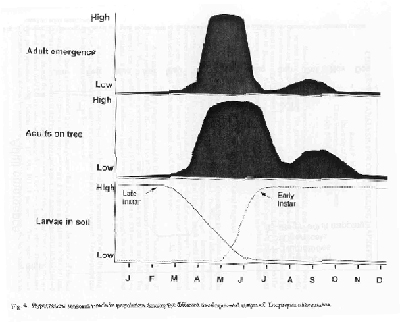
According to Tedders trap monitoring of adult Diaprepesemergence in central and east coast commercial citrus groves, peak emergence usually occurs over a 12-week period in the spring beginning in mid-March with a significant decline in mid-June (Fig. 1). Adults emerge in low number throughout the year and can generate a smaller peak in late-August through September. Since the adult female life span is about 12 weeks, she could presumably survive and lay eggs into late-summer. Assuming that peak adult emergence is reached in early May, the highest number of adults active within the tree will be during May through July (Fig. 4). Because adults are most abundant at this time of the year, it is safer to assume that oviposition is also at a high level at this time when food is abundant. Because eggs require about 8-10 days to hatch at 78o F, we can assume that maximum neonate invasion into the soil is occurring well into the summer (Fig. 4). Soil temperatures during the spring/summer period suggest that larva development on roots requires about 90 days through 10 instars. Therefore, when the 90 day period for maximum larval injury to the roots is superimposed onto the peak period of adult activity in the field, it would appear that maximum larval injury to the roots, by overlap of all instars, begins in early May and continues through July and August unless natural control becomes limiting. Previous research suggests that highest larval parasitism by native entomopathogenic fungi occurs in the spring and parasitism by entomopathogenic nematodes is highest from July to mid-October.
In a previous section, the various chemical and non-chemical tools available for combating larval and adult population were discussed. According to our limited understanding of weevil biology as previously outlined, how can we use the few control options most effectively:
- Although foliar sprays for adult control are very limited in term of residual control and no data is available on their effect on subsequent weevil generations following their use during the previous year, they do kill a high percentage of adults for 2-3 weeks or inhibit viable egg production by exposed females for a short time (Fig. 5). If growers choose to use a foliar spray for these purposes, it is imperative that treatments are made in the May through June when adults are highest. Two applications of a foliar treatment are suggested, but no more. Both Guthion and Sevin can incite abnormal increase in spider mite populations and any pesticides, when used frequently, will cause secondary pest outbreaks or could lead to resistance.
- The use of Capture as a soil barrier treatment must focus on the period of the year when neonate invasion of the soil is most prevalent (Fig. 5). Biological information on the weevil suggests that application in mid-April or early May will maximize control since residual effect during this time of the year is 10-12 wk. Since the compound remains on the soil surface, it should have little effect on natural enemies found in the soil.
- The use of Bio-Vector 355 as a treatment for all developmental stages of Diaprepes in soil should focus on the summer and early fall period to minimize root injury and take advantages of the higher level of soil moisture at this time of the year (Fig. 5). Since nematodes applied commercially are persistent for 4 wk or less, multiple applications are likely to give the best results. Like foliar sprays, limited residual requires multiple sprays and in the case of nematodes, cost to the grower is limiting. Nematode applications are restricted by soil temperatures below 80o F, so application in the winter should be avoided and spring application should be with caution.
- A combination of foliar, chemical and nematode applications or chemical and nematode applications only at the above times of the year suggest the best control, but costly to apply.
In addition, a number of factors should be considered when making decisions to control adults and/or larvae of Diaprepes. Weevil identification should be confirmed. The age of the citrus planting and rootstock should be a consideration. Young trees with smaller root systems can not tolerate the same level of adult and larvae feeding as those of mature trees and rootstock susceptibility to Phytophthora spp. can be a factor.
A program of frequent, light application of fertilizer and water can be used to regenerate replacement fibrous roots and compensate for structural roots damaged by D. abbreviatus, but Phytophthora spp. may continue to damage structural roots. When soil sampling detects populations of Phytophthora spp. in Diaprepes-infested groves, fungicide applications should be considered to protect structural roots of susceptible rootstocks. Under most conditions, populations of the fungus must reach 10-20 propagules per cm3 soil before considering a treatment program. In the presence of D. abbreviatus, soil populations may underestimate structural root damage by Phytophthora spp. in weevil-affected groves because the assay measures fibrous root rot activity.
Management in Citrus Nurseries
Because of their broad host range, Diaprepes root weevil will infest many container-grown ornamentals as well as all citrus varieties. Since adults disperse slowly in the field, Diaprepes have historically been disseminated between or within countries as eggs or adults on plant foliage or as larvae on the roots of container-grown nursery plants.Diaprepesroot weevil management in nurseries begins with purchasing plant material that is certified weevil-free and maintaining a weevil-free nursery. It is important to inspect the canopy of all plants for leaf injury, eggs and adults. In addition, container-grown plants showing symptoms of decline should be bare-rooted and examined for larvae and feeding injury.
Sanitation is vital to any nursery. Greenhouses and shadehouses must be kept insect-proof through careful preventive maintenance. The following management program is recommended for either enclosed or outdoor nurseries subject to weevil invasion from the surrounding area. Plants should be inspected weekly for adult weevils. In outdoor nurseries, a few ground traps, such as the pyramid-shaped Tedder's trap, can be placed on the soil surface at random locations near the base of suspected host plants, to attract adults moving on the soil. Foliar chemical sprays should be applied as needed to kill adult populations on the foliage at the time of detection and thereby reduce the number of gravid females laying eggs on the plant. In nurseries with a history of weevils, soil insecticides should be applied as a liquid drench or as granules fully incorporated into the potting mix for the control of first-instar larvae invading the soil. Residual control lasts a minimum of 10 weeks but has no effect on larvae beyond the first instar.
Acknowledgements
We wish to thank Angel Hoyte for her assistance with manuscript preparation, Jerry Fojtik and Ian Jackson for technical assistance. This research was supported in part by a grant from the Florida Citrus Production Research Advisory Council.
References
1. Bullock, R. C., C. W. McCoy, and J. Fojtik. 1988. Foliar sprays to control adults of the citrus root weevil complex in Florida. Proc. Fla. State Hort. Soc. 101:1-5.
2. Diaprepes Task Force. 1997. Diaprepes root weevil fact sheet. Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Bureau of Pest Eradication and Control, Gainesville, FL.
3. Duncan, L. W., D. I. Shapiro, C. W. McCoy, and J. H. Graham. 1999. Entomopathogenic nematodes as a component of citrus root weevil IPM. In "Optimal Use of Insecticidal Nematodes in Pest Management" (S. Polavarapu, Ed.), pp. 69-78. Rutgers, University, New Brunswick, NJ.
4. Graham, J. H., C. W. McCoy, and J. S. Rogers. 1996. Insect-plant pathogen interactions: Preliminary studies of Diaprepes root weevil injuries and Phytophthora infections. Proc. Fla. State Hort. Soc. 109:57-62.
5. Knapp, J. L. 1999. Florida Citrus Pest Management Guide. Cooperative Extension Service - IFAS, SP-43, Gainesville.
6. McCoy, C. W. 1999. Arthropod pests of citrus roots. In "Citrus Health Management" (L. W. Timmer and L. W. Duncan, Eds.), pp. 149-156. APS Press, St. Paul, MN.
7. Quintela, E. D., J. Fan, and C. W. McCoy. 1998. Development of Diaprepes abbreviatus (Coleoptera: Curculionidae) on artificial and citrus root substrates. J. Econ. Entomol. 91:1173-1179.
8. Schroeder, W. J. Diflubenzuron residue.: Reduction of Diaprepes abbreviatus (Coleoptera: Curculionidae) neonates. Fla. Entomol. 79(3):462-463.
9. Whitcomb, W. H., T. D. Gowan, and W. F. Buren. 1982. Predators of Diaprepes abbreviatus larvae. Fla. Entomol. 65(1):150-158.
10. Wolcott, G. N. 1936. The life history of Diaprepes abbreviatus at Rio Piedras, Puerto Rico. J. Agric. Univ. P. R. 20:883-914.
Table 1. Root weevils known to reproduce on and damage citrus.
| Genus | Common name | Geographical location |
|---|---|---|
| Major pests1 | ||
| Asynonychus | Fuller rose beetle | United States, Australia, New Zealand, Chile, Argentina |
| Diaprepes | Diaprepes root weevil | United States, West Indies |
| Exophthalmus | Fiddler beetle | West Indies |
| Naupactus | -- | South America |
| Pachnaeus | Blue-green citrus weevil | United States, West Indies |
| Pantomorus | -- | Central America, South America |
| Minor pests | ||
| Artipus | Little leafnotcher | United States, West Indies |
| Campsus | -- | Central America, West Indies, Texas |
| Cleistolophus | -- | West Indies |
| Epicaerus | -- | Mexico, Central America, Florida, Texas |
| Eutinophaea | Citrus leaf-eating weevil | Australia |
| Lachnopus | -- | West Indies |
| Litostylus | -- | West Indies |
| Maleuterpes | Spine-legged citrus weevil | Australia |
| Orthorhinus | Elephant weevil | Australia |
| Tanymecus | -- | North America |
| 1Major pests known to cause significant economic losses to citrus in more than a single, isolated region of the world. | ||